We develop, manufacture and market human and veterinary pharmaceuticals and active pharmaceutical ingredients. We believe that health and well-being create the foundation for a good life and society.
We are continuously developing new drugs and treatment methods. The core therapy areas of our pharmaceutical R&D are oncology and pain.
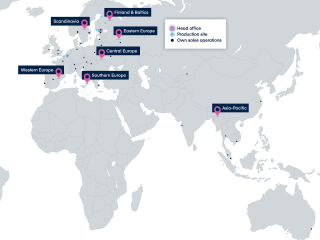
We collaborate closely with healthcare professionals and leading global research institutions and pharmaceutical companies.