Carbon Footprint Assessment of Orion’s Dry Powder Inhalers
In Europe 30 million people with asthma need to use different medications to treat asthma symptoms like shortness of breath or cough. Majority of medications are taken via inhalers, devices delivering either powder or aerosol formulation of drugs into the lungs. Developing and manufacturing these inhalers obviously have an environmental impact.
Orion conducted the carbon footprint assessment for the whole Easyhaler® product range in 2021(1). This is a continuum for the initial life cycle assessment (LCA) performed in 2019 for a selection of Easyhaler products (2). According to this newest assessment, the average carbon footprint of one inhaler is 0.580 kg CO2e.
We wanted to improve our understanding of the climate impact and more comprehensively of the environmental impact of our Easyhaler product range. LCA is the tool fit for that purpose. It looks at the environmental impacts made by a product over its whole life, usually broken into different stages, such as raw material extraction, manufacture, distribution, use and disposal. LCA is a tool to give robust information on the environmental impacts of a product but also to identify where reduction efforts should be focused.
Our first LCA in 2019 was conducted for a dry powder inhaler Easyhaler, for the treatment of asthma and chronic obstructive pulmonary disease (COPD). Easyhaler is one good example of our efforts since 1980’s for the environment as one of the original Easyhaler development objectives was to create a propellant-free inhaler for environmental reasons. The Montreal Protocol in 1987 prohibited the use of ozone-depleting chlorofluorocarbon (CFC) propellants for inhaled products as used in metered dose inhalers (MDIs). Since the Montreal Protocol came into force, the use of most ozone-depleting substances has either ceased or at least declined. CFCs were replaced by hydrofluoroalkane (HFA/HFC) propellants, and their use is still permitted in MDI medications. In MDIs, propellants are discharged during use and released after product disposal. These propellants, HFCs, are potent greenhouse gases with around 1300 times more global warming potential than carbon dioxide. And, the carbon footprint of single MDI products is still 10-37 times higher than dry powder inhalers (DPIs) (3). Based on earlier assessments, DPIs have been reported to have carbon footprint between 1.5 and 6kg CO2e for 200-doses inhaler (4).
The cradle–to-grave LCA was conducted for the full Easyhaler product range including six products available for the treatment of asthma and COPD (budesonide-formoterol, salmeterol-fluticasone, salbutamol, formoterol, budesonide and beclomethasone Easyhaler). For each, the most used strength and number of doses per device was used for the assessment. The analysis covered raw material extraction, upstream materials preparation, transportation, manufacture, processing and assembly, distribution and disposal. The life cycle inventory data is based on our own measurements and data, collected from suppliers, and internationally available life cycle inventory databases. Analyses were performed according to ISO 14040 LCA standard series by a certified independent third party, Carbon Footprint Ltd.
The results represent the estimated environmental impact for creating, using and disposing the inhaler device. Several environmental indicators, such as climate change, toxicity and water depletion were assessed to ensure holistic understanding on the environmental impact. The most important source of impact for most of the indicators was from manufacture, which accounted for approx. 63% (range 55-67%) for carbon footprint. In comparison, emissions from distribution accounted for less than 2%, indicating that most potential for improvement lies in manufacture processes. It is also noted that the results of a life cycle assessment (LCA) always depend on the calculation method, scoping and assumptions used and they reflect our understanding at the time when published.
Carbon footprint for one Easyhaler, 0.580 kg CO2e
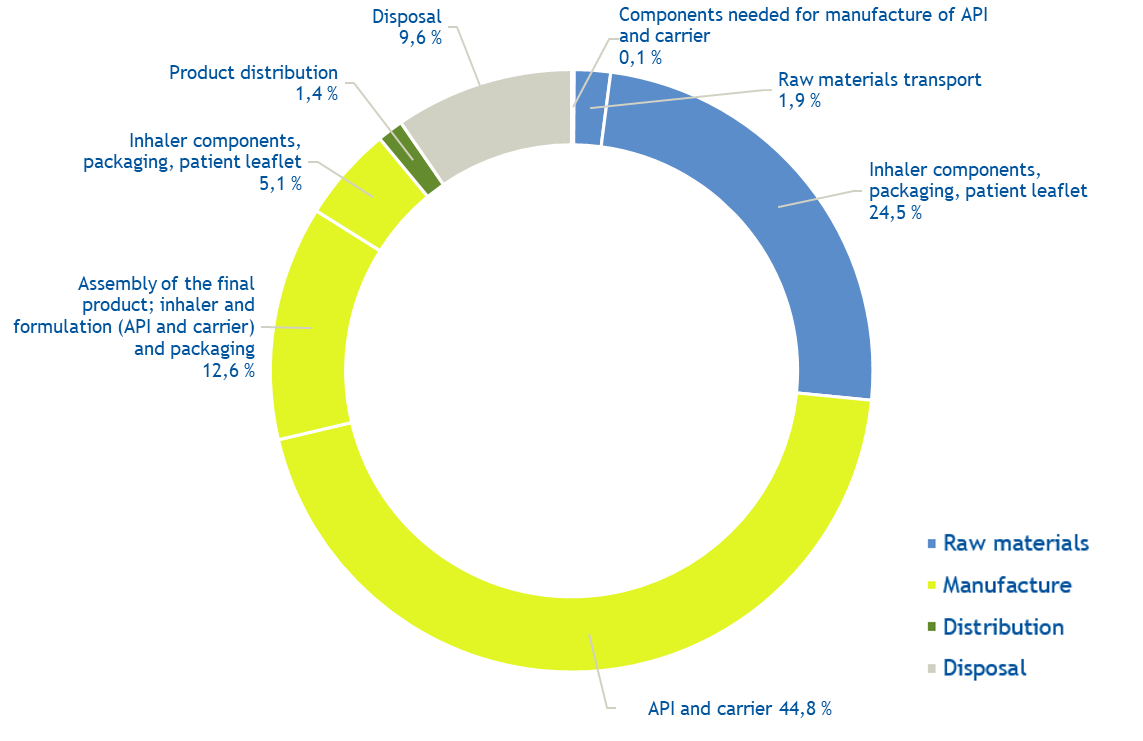
Average of the six Easyhaler products, range 0.484-0.650 kg. For each, the most used strength and number of doses per device was used for this analysis.
API: Active Pharmaceutical Ingredient
The efficacy and safety of treatment for patients’ diseases are of uttermost importance and the environmental aspects are something we increasingly strive to take into consideration. We focus on the entire product life cycle to reduce environmental impacts for example by driving energy savings, switching to entirely green electricity at factories and improving wastewater management. We focus our efforts also to ensure the responsible supply chain. Patients with asthma or COPD and treating physicians alike are increasingly aware of climate change and this affects even their treatment choices. When considering the climate impact of inhalation therapy, DPIs have a minimal carbon footprint compared with MDIs.
This LCA was used as a basis for offsetting the emissions of Easyhaler product range by supporting programs that remove the same amount of carbon dioxide that is emitted into the atmosphere over product life cyle. Healthcare professionals may register and explore more about carbon neutrality of Easyhaler at wehale.life.
Executive summary of Orion's Carbon Life Cycle Assessment Report
1. Product Carbon Footprint: Life Cycle Assessment Report for Orion Corporation, Orion Pharma. A study of 6 varieties of Easyhalers. Carbon Footprint Ltd 2021.
2. Product Carbon Footprint: Life Cycle Assessment Report for Orion Corporation, Orion Pharma. A study of 4 varieties of Easyhalers. Carbon Footprint Ltd 2020. Executive summary.
3. Medical and Chemicals Technical Options Committee. 2018 Assessment Report. Available at: https://ozone.unep.org/science/assessment/teap/. [Accessed 13 February 2020].
4. Wilkinson AJK et al. BMJ Open 2019;9:e028763.doi:10.1136/bmjopen-2018-028763








